E. chromi from Alexandra Daisy Ginsberg on Vimeo.
Field of Science
-
Change of address2 months ago in Variety of Life
-
Change of address2 months ago in Catalogue of Organisms
-
-
Earth Day: Pogo and our responsibility4 months ago in Doc Madhattan
-
What I Read 20245 months ago in Angry by Choice
-
I've moved to Substack. Come join me there.7 months ago in Genomics, Medicine, and Pseudoscience
-
-
-
-
-
Histological Evidence of Trauma in Dicynodont Tusks6 years ago in Chinleana
-
Posted: July 21, 2018 at 03:03PM7 years ago in Field Notes
-
Why doesn't all the GTA get taken up?7 years ago in RRResearch
-
-
Harnessing innate immunity to cure HIV9 years ago in Rule of 6ix
-
What kind of woman would pray for health or use spiritual healing?9 years ago in Epiphenom
-
-
-
-
-
-
post doc job opportunity on ribosome biochemistry!10 years ago in Protein Evolution and Other Musings
-
-
Blogging Microbes- Communicating Microbiology to Netizens10 years ago in Memoirs of a Defective Brain
-
Re-Blog: June Was 6th Warmest Globally11 years ago in The View from a Microbiologist
-
-
-
The Lure of the Obscure? Guest Post by Frank Stahl13 years ago in Sex, Genes & Evolution
-
-
Lab Rat Moving House14 years ago in Life of a Lab Rat
-
Goodbye FoS, thanks for all the laughs14 years ago in Disease Prone
-
-
Slideshow of NASA's Stardust-NExT Mission Comet Tempel 1 Flyby14 years ago in The Large Picture Blog
-
in The Biology Files
Coloured bacteria vs. Angry birds
Targeting dormant bacteria
Rather than targeting metabolic enzymes, the current strategies being explored to combat dormant bacteria target either the membrane, or membrane bound proteins. Both of these approaches destabilise the bacterial membrane and help to break the cell apart and can act against processes such as energy synthesis which occur in both active and dormant cells.
 a=targeting important metabolic proteins in the membrane. b=targeting the actual cell-membrane. Picture is copywrite me :p
a=targeting important metabolic proteins in the membrane. b=targeting the actual cell-membrane. Picture is copywrite me :p Drug developed to help combat TB by attacking cell membrane metabolic enzymes. This drug is currently in stage three clinical trials.
Drug developed to help combat TB by attacking cell membrane metabolic enzymes. This drug is currently in stage three clinical trials.There’s something about those molecular diagrams of drugs that I love. I think it’s my biochemical background. I’m never totally happy with a schematic until I can see how the chemicals are interacting on a molecular scale.
As well as being useful against dormant bacteria these new antimicrobials show promise as strategies for dealing with arising antibiotic resistance. Bacteria can evolve to cope with as many challenges as are thrown at them, but hopefully it should take them a little longer learn to survive entirely without a cell wall…
Although there are some that can do that already.
---
Hurdle JG, O'Neill AJ, Chopra I, & Lee RE (2011). Targeting bacterial membrane function: an underexploited mechanism for treating persistent infections. Nature reviews. Microbiology, 9 (1), 62-75 PMID: 21164535
---
Follow me on Twitter!
Antibiotics and gut bacteria
All microbiologists end up writing about gut bacteria at some point. It is the way of things. Disease of the Week is currently doing a whole series on it, and a few weeks ago I covered the interaction of the immune system with gut bacteria (here). However a recent paper came out in Microbiology Today concerning the affect of antibiotics on gut bacteria, which is a topic that I both find interesting and have had some actual experience with.

If you have taken antibiotics it's not worth loosing sleep over, but it's something hospitals are starting to be more and more aware of.
---
Chopping bits out of the genome
Streptomyces bacteria, however, have bigger genomes and the luxary to invest in genes which are not strictly necessary for bacterial survival. These are called Secondary metabolite genes (as opposed to the necessary primary metabolites) and they code for genes that form an arsenal of weapons for the Streptomyces to deploy. Most Strep are soil-based, and they need the ability to produce secondary metabolites (such as antibiotics) to fight off invading bacteria, and clear terratory to expand their growth into.
What has recently been done (very ingeniously) is to remove the secondary metabolism genes from the bacterial species Streptomyces avermitilis creating essentially an 'empty' strep bacteria, that can grow and divide but not produce any of the secretory substances that strep are known for. The researchers managed to cut an entire 1.5Mb of DNA right out of the genome - helped by the fact that all the secondary metabolite genes cluster together on one side of the chromosome.
They did this using a common molbio technique, the cre-lox system. "Lox" is an area of DNA and "cre" is the protein that very specifically cuts DNA at the Lox site - it acts like a pair of scissors. They put a Lox site on either end of the DNA that codes for secondary metabolites (using a techinque called recombination in order to attach the lox into the chromosome) along with the DNA for the Cre protein under an inducible promoter. Once the bacteria had grown, they activated Cre production, which then cut the unwanted DNA out of the bacteria. This technique was amazingly successful and is shown diagramatically below (picture from the reference):

The avimitilis now contains no secondary metabolites at all, which makes a wonderful 'empty' system to use for studying how secondary metabolites are made. Genes from other bacteria, or even some of the removed genes, can be added back in, piece at a time, to see how much of the gene is necessary for metabolite production, and which regulatory pathways are the most important. Creating an empty cell also has potential implications for biotechnology, after all when trying to produce antibiotics you want the cell working as hard as possible just to produce your product, not wasting time and resources on other metabolites!
I'm really impressed at this kind of large scale synthetic-biology. It may be an area I end up going into in the near future...
---
Komatsu M, Uchiyama T, Omura S, Cane DE, & Ikeda H (2010). Genome-minimized Streptomyces host for the heterologous expression of secondary metabolism. Proceedings of the National Academy of Sciences of the United States of America, 107 (6), 2646-51 PMID: 20133795
How bacteria die - SGM series
Unlike multicellular organisms, bacteria have no surrounding restraints on their mutation rate - with the exception of bacteria in aggregates the only thing a bacteria will harm by changing it's DNA is itself. This gives bacteria a lot more genetic plasticity. Added to this, changing DNA is one of the main ways bacteria go about improving themselves, and adapting to new conditions. Changing the DNA by wholescale random mutagenesis is a bit extreme, but if you're in a stressful situation anyway it might be worth a shot.
---
Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, & Collins JJ (2007). A common mechanism of cellular death induced by bactericidal antibiotics. Cell, 130 (5), 797-810 PMID: 17803904
Ivana Bjedov, Olivier Tenaillon, Bénédicte Gérard, Valeria Souza, Erick Denamur, Miroslav Radman, François Taddei, Ivan Matic (2003). Stress-Induced Mutagenesis in Bacteria Science, 1404-1409 DOI: 10.1126/science.1082240
---
Follow me on Twitter!
Nanofibre paint that kills MRSA
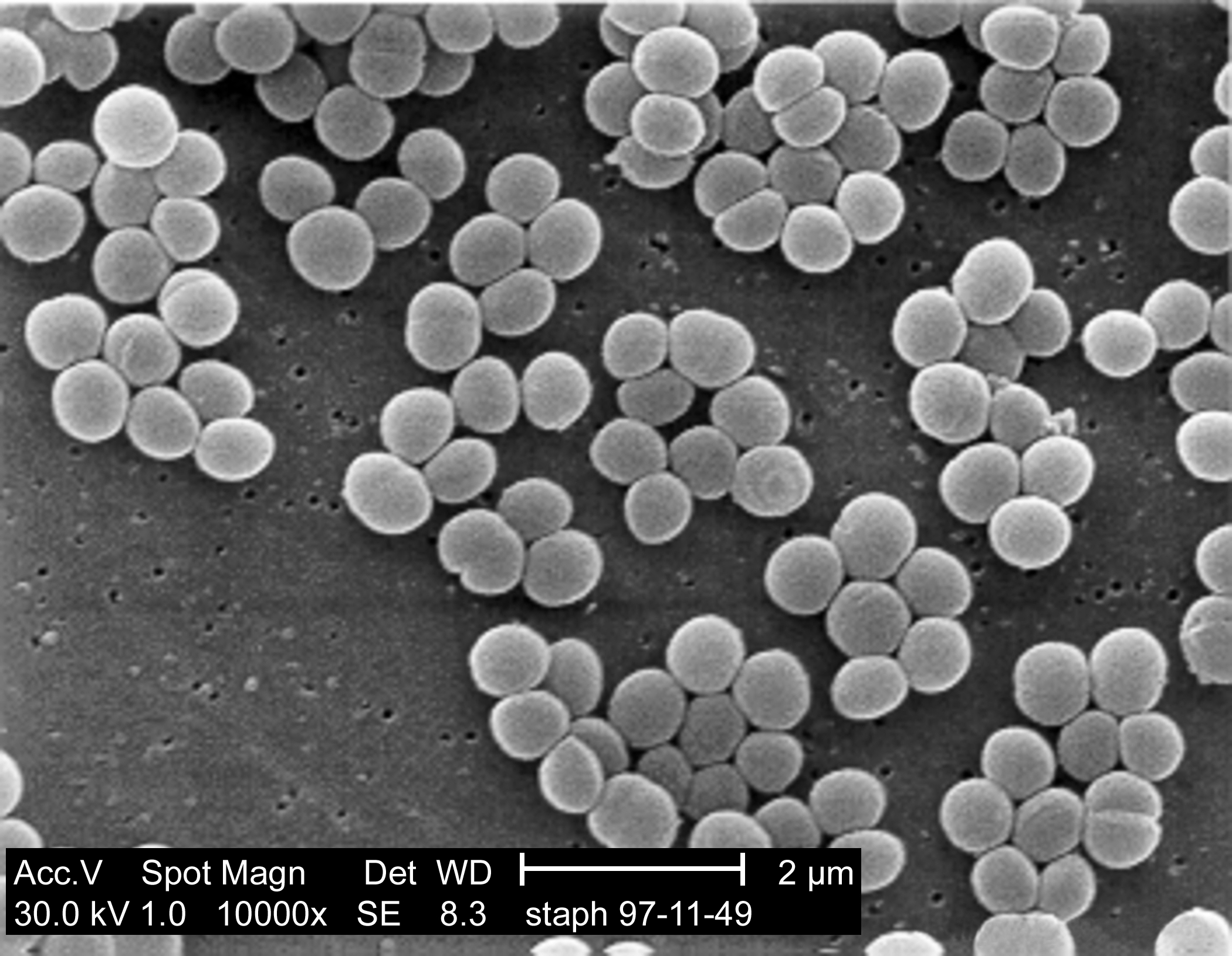
However medical scientists aren't the only ones trying to kill bacteria, virus's known as bacteriophages are also interested in breaking open bacterial cells and they do it using a cocktail of different enzymes to break open the cell wall. Many of these enzymes feature a two-domain structure with bacteria-specific cell wall targeting and catalytic domains. The enzymes Lst was found to be particularly good at breaking apart Staph aureus cell walls with one end of the enzyme (C terminus) recognising and binding to the bacterial cell wall while the other end (the N terminus) breaks the protein bridges between the sugar componants of the peptidoglycan layer, which is a major componant of the cell wall.
Work from the Rensselaer’s Center for Biotechnology has been looking at incorporating these enzymes into nanofibres to create stable bactericidal paint films. The molecular-level curvature of carbon nanotubes stabilizes a wide range of enzymes and the lab was able to successfully create Lst-containing nanocomposite films which achieved >99.9% killing of MRSA upon contact within 2 h. They also explored incorporating these into a latex paint, which retained the bactericidal properties of the nanofibres. This paint could theoretically be spread over hospital surfaces to reduce the numbers of Staph aureus within the hospital environment. Incorporating this enzyme into the nanofibres (rather than directly mixing with the latex) gives added stability and helps the enzyme stay within the coating for longer. Films which were stored dry at room temperature showed >99% bactericidal activity against S. aureus after 30 days.
It remains to be seen how effective this technique will be outside of a laboratory setting but at the moment it looks like a highly promising step to help reduce the incidence of a dangerous pathogen. The speed and likelihood of resistance also remains to be seen, but it's heartening that bacteriophages have been using these enzymes against the bacteria for far longer than we've been using antibiotics. This is unlikely to be any kind of magical anti-MRSA cure, but it could certainly be very useful in helping to reduce the incidence of the disease.
---
Pangule RC, Brooks SJ, Dinu CZ, Bale SS, Salmon SL, Zhu G, Metzger DW, Kane RS, & Dordick JS (2010). Antistaphylococcal nanocomposite films based on enzyme-nanotube conjugates. ACS nano, 4 (7), 3993-4000 PMID: 20604574
---
Follow me on Twitter!
Models for the evolution of bacterial resistance

---
Defoirdt T, Boon N, & Bossier P (2010). Can bacteria evolve resistance to quorum sensing disruption? PLoS pathogens, 6 (7) PMID: 20628566
Quorum Sensing and Biofilms

---
Nadell CD, Xavier JB, Levin SA, & Foster KR (2008). The evolution of quorum sensing in bacterial biofilms. PLoS biology, 6 (1) PMID: 18232735
Antibiotics and Synthetic Biology
---
Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, & Collins JJ (2007). A common mechanism of cellular death induced by bactericidal antibiotics. Cell, 130 (5), 797-810 PMID: 17803904
Kohanski MA, Dwyer DJ, & Collins JJ (2010). How antibiotics kill bacteria: from targets to networks. Nature reviews. Microbiology, 8 (6), 423-35 PMID: 20440275
Two Component Systems

Diagram drawn by me, using all the MS Paint skill I possess. (I've tried to keep the colours colour-blind friendly). Sensor in green, responder in blue, and the brown lines show the path of the phosphate. Blob on the left is the signal molecule that the system is sensing. 'H' and 'D' are amino acids Histadine and Aspartate respectively.
One of the most useful things about this system from a scientific point of view is that the phosphorylated regions are very well conserved across bacterial species. This makes them relatively easy to find, once you have the full genome of the organism, as shown by large-scale searches for two component systems in Bacillis subtilis and Streptomyces coelicolor (both references given below). In both organisms hidden Markov models were used to find the conserved protein sequences, and then sequence alignments carried out to group the sensors and responders into different groups. They also searched for transmembrane domains within the sensors to find whether (and how) they were attached to the surface of the bacterial cell. Unattached soluble sensors suggest a monitoring of the intracellular environment, whereas membrane bound sensors are more likely to provide information about external conditions.
As the function of many of the two component systems (particularly in Strep. coelicolor) is unknown, studies like this provide exciting new avenues of research to explore. One of the main commercial attractions to studying two component systems (ignoring the main attraction, which is simply to find out how the things work) is that they aren't present in animal cells, and therefore could potentially be a target for novel antibiotics. Particularly as many of them are vital for the survival of the bacteria, particularly opportunistic motile pathogens.
In fact, two component systems are very often the way the bacteria senses and responds to the antibiotics as well. Knocking out the vancomycin response system (VanRS) might not kill the bacteria, but combining it with vancomycin treatment would be deadly.
---
Work is sort of getting to be at the moment, now term has properly started there seems to be an awful lot of it happening, and everything is going slightly crazy. So if anyone would like to write guest posts (both blog-owners and non-blog-owners) they would be happily recieved. Leave some form of contact in the comment box (or write to me for those who know my email address) and I'll get back to you.
All I ask is that the posts be vaguelly about science. :)
---
Fabret C, Feher VA, & Hoch JA (1999). Two-component signal transduction in Bacillus subtilis: how one organism sees its world. Journal of bacteriology, 181 (7), 1975-83 PMID: 10094672
Hutchings MI, Hoskisson PA, Chandra G, & Buttner MJ (2004). Sensing and responding to diverse extracellular signals? Analysis of the sensor kinases and response regulators of Streptomyces coelicolor A3(2). Microbiology (Reading, England), 150 (Pt 9), 2795-806 PMID: 15347739
Biofilms and Bioshields
 Pseudomonas aeruginosa is an opportunistic human pathogen that can form biofilms and is often used to study biofilm formation. It can survive in soil, on the skin, and on a variety of surfaces, including hospital equipment such as catheters. It tends to infect humans who are already ill, and can colonise the urinary tract, kidneys and lungs, the latter making it a major secondary complication in cystic fibrosis.
Pseudomonas aeruginosa is an opportunistic human pathogen that can form biofilms and is often used to study biofilm formation. It can survive in soil, on the skin, and on a variety of surfaces, including hospital equipment such as catheters. It tends to infect humans who are already ill, and can colonise the urinary tract, kidneys and lungs, the latter making it a major secondary complication in cystic fibrosis.One of the defenses against lung bacteria are polymorphic neutrophilic leukocytes (a type of bacteria-eating white blood cell, hereafter referred to as PMNs). These produce a variety of antibacteria chemicals, which break down invading species. However they are not able to destroy the P. aeruginosa biofilms. This isn't just because the biofilm is too complex to break into, it's due to a specific chemical; rhamnolipid. Remove the rhamnolipid from the bacteria and the PMNs can happily munch through the biofilm. This was shown with in vivo mouse studies, animals infected with bacteria unable to produce rhamnolipid contained twice as many viable PMNs in their lungs.
However rhamnolipids are not expressed all the time. They are quite potent chemicals after all, and could disrupt the surface of the cells that the P. aeruginosa are trying to grow on. They are only expressed in the presence of PMNs, as shown in the graph: (figure adapted from the reference below).

The dots on the very left show wild-type P. aeruginosa cells exposed to PMNs. Next, just the wild type cells, without any PMN exposure. These produce a lot less rhamnolipid. The final two results are taken from cells which are unable to produce any rhamnolipid, due to a gene knockout.
One of the most interesting things about the rhamnolipids is that they are not secreted outside the biofilm. Rather than release them into the surrounding environment the bacteria holds onto them, using them as a shield to coat the biofilm and protect it from PMN attack. Microscopy studies show that this is where the PMNs are killed as well, on the outside surface of the biofilm. This allows the action of a potentially destructive attack chemical to be carefully controlled. The rhamnolipid is only produced by the bacteria when a specific threat is present, and will then only act as a defense against cells that specifically try to attack the biofilm (microscopy figure taken from the reference below):

The biofilm is stained pink, while the surrounding PMN cells are stained blue, showing a clear dividing line between the bacteria and the surrounding attacking cells. The blue stains DNA, rather than cells, so it is the nuclei of the cells that are visible. In the top left of the picture, near the biofilm, some fuzzy blue lines can be seen, rather than the better defined blue blobs; these are DNA from PMNs that have been lysed and killed by the rhamnolipids.
The importance of this work lies in the fact that antibiotics don't really work on biofilms. Biofilms are made up of many layers of bacteria, packed deep in a peptidoglycan matrix. The antibiotic can kill off bacteria near the top of the biofilm, but can't penetrate deep enough to kill all the bacteria near the bottom. As these bacteria are exposed to a lower concentration of antibiotic (i.e not enough to kill them) they are also a lot more likely to develop resistance.
Researching interactions with the immune system therefore provides more information about new potential mechanisms for removing the bacterial infection. This is especially important for diseases like P. aeruginosa which hang around in hospitals, and are a major risk of secondary infection.
---
Alhede M, Bjarnsholt T, Jensen PØ, Phipps RK, Moser C, Christophersen L, Christensen LD, van Gennip M, Parsek M, Høiby N, Rasmussen TB, & Givskov M (2009). Pseudomonas aeruginosa recognizes and responds aggressively to the presence of polymorphonuclear leukocytes. Microbiology (Reading, England), 155 (Pt 11), 3500-8 PMID: 19643762
Lab etiquette and priorities...
So what do I do when (as happened just now) someone asks me if they can use the hood for the afternoon? And in a way that suggests that they kind of really need to use the hood that afternoon...
(Hood = laminar flow hood, which is a sterile working space).
What I did was to internally rearrange my schedule inside my head, to free up space for them. But what if that hadn't been an option? What if I'd been doing an infinitely-long colony pick or something? Who, in that situation, has the priority?
The PostDoc with their relevant research, or the Lab Rat who, by an accident of bureaucratic shuffling, happens to actually be in the lab that owns the hood? (there's about three labs in the space upstairs and we tend to share equipment)
I quickly finished up everything I needed to do right then, and then told the aforementioned PostDoc that she could use the hood whenever. Except...I still have more work to do in there this evening. And she hasn't actually started work in there yet. I don't want to start my work in case she suddenly appears, but I have no idea how long it is polite to wait before getting naggy at someone. Especially when that someone is a) older than me b) doing more important work for the lab and c) bringing more money in for the lab.
I have decided to deal with this problem in my usual way of dealing with all problems, by scuttling away and hoping it disappears in it's own time if I stop looking at it. Which is why I'm currently sitting downstairs in the dry lab using the computer and consoling myself with vending machine chocolate bars. I can't really go up until either she's finished with the hood or my E. coli plates dry, and they won't for a while because I accidentally massively over-poured one of them.
On the plus side, I discovered why my transformations didn't work yesterday. I plated them out on the wrong antibiotics and they all died. In my current state of mind, I can't help but find this kindof hilarious.
Damage Response Systems
Firstly, a specific response against the attacking antibiotic. These can take the form of antibiotic degrading-enzymes, or efflux pumps, which move the antibiotic out of the cell. In the case of bacitracin, it's an efflux pump (encoded by the bcrABC cassette), which uses energy from ATP to transport the bacitracin out of the cell.
The most interesting thing about this system, and indeed many of the antibiotic-specific response systems, lies in it's evolutionary origins. The cassette originally came from a bacteria called Bacillus licheriformis, which is the bacteria that makes the bacitracin antibiotic in the first place. Soil bacteria tend to make a huge number of antibiotics, for defense and invasion, and if you make an antibiotic, it's a good idea to have some way of ensuring that it doesn't destroy your own cellular systems. In fact, given that this is an efflux pump, it might not even have evolved as a defense mechanism...just a pathway for moving the bacitracin into the environment once it had been made, as it is a secreted antibiotic
These ABC transporter systems are found fairly frequently as well. In B. subtilis (one of the better studied bacillus bacteria) eight out of the forty antibiotic genes have ABC transporter systems next to them. Because unlike in eukaryotes (like people) who can often have genes for similar systems on wildly different parts of the chromosome, bacteria like to keep genes used for the similar functions close together. They don't have much genome, they don't have the space pr the protection of a nuclear cell membrane, so they have to be more efficient about packaging.
The second type of response is a more generalised system; rather than responding to a particular antibiotic, it is instead a cellular response to the damaged cell wall. As an example the LiaRS system (a two-component response system)is activated in response to four different cell wall attacking antibiotics (all of which interfere with the rate limiting step of cell-wall building, the lipid II cycle). The Sensor (LiaS) has a short histadine kinase domain which is buried in the membrane. This recognises membrane damage and uses the energy from ATP to phosphorylate the Response Regulator (LiaR) which then leads to gene activation.
The Lia system is more than just a two component system however, there is a third component. As well as the sensor and responder, there is a third protein LiaF which keeps the system 'switched off' when the cell wall is not damaged. This is shown diagrammatically below:

Image from second reference (Jordan et al 2006)
When the cell wall is damaged, the LiaF inhibition is removed, and the LiaS can phosphorylate the LiaR, leading to a change in gene expression, which produces the appropriate response.
Unlike the specific responses, these pathways are often present within the bacteria, as a natural response to cell wall damage. These are not so much resistance mechanisms, as survival mechanisms, that are strongly selected for in times of antibiotic stress. The damage caused by clinical concentrations of antibiotic is usually too much for such systems to cope with, but they form an adequate defense against antibiotic levels in the soil.
--
Ohki, R., Tateno, K., Okada, Y., Okajima, H., Asai, K., Sadaie, Y., Murata, M., & Aiso, T. (2003). A Bacitracin-Resistant Bacillus subtilis Gene Encodes a Homologue of the Membrane-Spanning Subunit of the Bacillus licheniformis ABC Transporter Journal of Bacteriology, 185 (1), 51-59 DOI: 10.1128/JB.185.1.51-59.2003
Jordan, S., Junker, A., Helmann, J., & Mascher, T. (2006). Regulation of LiaRS-Dependent Gene Expression in Bacillus subtilis: Identification of Inhibitor Proteins, Regulator Binding Sites, and Target Genes of a Conserved Cell Envelope Stress-Sensing Two-Component System Journal of Bacteriology, 188 (14), 5153-5166 DOI: 10.1128/JB.00310-06
How to destroy a bacterial cell wall...
Bacterial cell walls are made of strands of glycopeptide (shown below, picture from Kimball's biology pages) crosslinked together to form a mesh, which provides a strong support around the cell. There's a whole pathway of enzymes involved in creating the structure, and blocking them, or preventing them from working efficiently, is a quick and easy way of killing off bacteria.

This is the strategy used by Methicillin, an antibiotic that used to be talked about a lot a while ago (it's the 'M' in MRSA) but has been neglected by the media lately in favour of swine-flu and other viruses. Methicillin is a B-lactam antibiotic, which means that it binds to one of the enzymes involved in cell wall metabolism, blocking its active site. More specifically, it binds to the enzyme that creates the cross-links between the glycopeptides (PBP2). No new cell wall can be created, and therefore no more bacteria.
Resistance to this takes several forms. MRSA simply uses a variant of the enzyme, with a deeper active site, so that while the cell-wall precursor substrate can bind, the antibiotic cannot. Protection from a wide variety of different B-lactams can be achieved by B-lactamases, bacterial enzymes which break down the antibiotics. Multi-efflux pumps also exist, these are proteins that span the bacterial cell wall and essentially pump out any antibiotics that make their way into the cell before they can cause any harm.
Vancomycin is the drug that is still most commonly used against MRSA, although some resistance is (as always) beginning to arise. Unlike methicillin, vancomycin does not bind to any bacterial enzymes, instead it binds directly to the cell wall precursors. The part it binds to is shown below, as a close up from the earlier diagram of the cell wall:
 The incredibly inexpertly added D-ala in red at the bottom shows the precursor form of this section of the cell wall (Ala, Glu and Lys are the short-hand form of amino-acids, so this is just a short protein chain. The L- D- labels show what form the amino-acids are in). The vancomycin binds to the final D-ala-D-ala, preventing it from being processed and halting construction of the cell wall.
The incredibly inexpertly added D-ala in red at the bottom shows the precursor form of this section of the cell wall (Ala, Glu and Lys are the short-hand form of amino-acids, so this is just a short protein chain. The L- D- labels show what form the amino-acids are in). The vancomycin binds to the final D-ala-D-ala, preventing it from being processed and halting construction of the cell wall.Two different methods of preventing cell wall growth; one antibiotic binding to the enzyme, the other to the substrate. And both, sadly, have been defeated by resistance already. In the case of the B-lactams, a wide variety of resistance mechanisms exist, from actively destroying the antibiotic, to pumping it out the cell, or bypassing the enzyme completely. In the case of vancomycin, some bacteria have started producing peptide chains ending in D-ala-D-ser, or D-ala-D-lac, and there have been reports of VRSA from America.
The pathway for creating bacterial cell walls contains multiple steps, and both methicillin and vancomycin halt just one of these, the cross-linking of the short peptide chains near the end. Bacitracin, another cell-wall directed antibiotic, works further upstream in the pathway. I've just been given a whole stack of papers on bacitracin by my supervisor...so I'll probably be writing more about that in the future!
Bacteria that use antibiotics...for food!
However researchers at Harvard found that not only are some bacteria able to neutralise the threat of antibiotic resistance, they actually use antibiotics as a food source. Not only that, but they were capable of using antibiotics as the sole carbon source. The table below (taken from the reference at the end of the post) shows the survival of bacteria on antibiotics using samples from three different types of soil, Farmland (F), Urban (U) and Pristine (P - soil from non urban areas with minimal human contact for 100 years):
 The antibiotics used include natural, synthetic and semi-synthetic molecules, all all of which could be used by bacterial species as a carbon source. Even more interestingly (or alarmingly) the antibiotics were at concentrations of 1g/litre, 50 times higher than the concentration normally used to test for resistance.
The antibiotics used include natural, synthetic and semi-synthetic molecules, all all of which could be used by bacterial species as a carbon source. Even more interestingly (or alarmingly) the antibiotics were at concentrations of 1g/litre, 50 times higher than the concentration normally used to test for resistance.The 'pristine' soil is the one that the researchers found the most interesting, as the general expectation was that this area would contain fewer antibiotic-eating bacteria, having had minimal interaction with people and pharmaceutical antibiotics. However the data showed no noticeable difference, despite not being in contact with human-designed antibiotics, the bacteria are meeting plenty of bacterial-based antibiotics, and adapting to use them for food.
The big question of course is Will it Spread? Around the quarters of the isolated strains belonged to orders containing clinically relevant strains such as Salmonella and E. coli, meaning that hypothetically at least antibiotic consumption should be able to spread. On the other hand, actual consumption of antibiotics is unlikely to provide a greater evolutionary advantage than just resistance, and will confer a larger metabolic load on the bacteria. Although the pathways of antibiotic metabolism have not yet been fully determined, the first few steps seem to be similar to well-known resistance mechanisms (particularly in penicillin consumption). One conclusion, therefore, is that only part of the metabolic pathway would be (or already has been) passed on to pathogenic organisms, enough to provide resistance without placing unnecessary metabolic burdens on the cell.
Hat tip to Byte Size Biology for alerting me to the paper.
--
Dantas, G., Sommer, M., Oluwasegun, R., & Church, G. (2008). Bacteria Subsisting on Antibiotics Science, 320 (5872), 100-103 DOI: 10.1126/science.1155157
Cell wall under attack - bacterial response to antibiotics
The bacterial cell wall is made up of glycopeptide molecules (sugars and proteins joined together) and surrounds the whole cell. Without it, bacteria swiftly loose their integrity and salt-balance across the membrane, which is why many antibiotics target the cell wall in order to kill bacteria. Both for antibiotic resistance, and for surviving conditions that could damage the cell wall, bacteria have a system of monitoring the state of the cell membrane and responding quickly to any changes.
The system that was discovered in Streptomyces coelicolor (which I'll be working on) was named the sigE system, and consisted of an operon (string of genes) encoding four genes:
 SigE encodes for a sigma-factor, a protein used in bacteria to switch on certain sets of genes. The cseA codes for a cell membrane lipoprotein, possibly used in a sensor system, while cseB and C are a two-component signalling system (very common in bacteria). CseC is a sensor (a histadine protein-kinase sensor for those who are interested) while cseB is the response regulator, acting out a response when it receives a signal from cseC.
SigE encodes for a sigma-factor, a protein used in bacteria to switch on certain sets of genes. The cseA codes for a cell membrane lipoprotein, possibly used in a sensor system, while cseB and C are a two-component signalling system (very common in bacteria). CseC is a sensor (a histadine protein-kinase sensor for those who are interested) while cseB is the response regulator, acting out a response when it receives a signal from cseC.And now...the science :)
In order to test that this operon was involved in cell membrane responses to antibiotics the lab carried out a variety of experiments, all producing evidence that lead towards this conclusion. The main experiments were as follows:
- Removing the sigE operon and placing it on a separate plasmid, that activated resistance to Kanamycin. The bacteria were then plated on agar containing antibiotics and challenged with a kanamycin disk. Cell wall attacking antibiotics induced kanamycin, whereas antibiotics that attacked (say) the ribosome didn't.
- Keeping the sigE in its original chromosomal context, the group then challenged it with different concentrations of vancomycin (an antibiotic which attacks bacterial cell walls). They then measured the level of the sigE operon proteins being produced in the cell. Higher concentrations of vancomycin, lead to more proteins.
- Going back to the sigE-kanamycin resistant protein, they tried knocking out the sigE promoter, effectively switching all these genes off. The effect seen previously disappeared.
- Leaving the lab, they then did some computational work, scanning the database to see what genes the sigE sigma-factor actually switched on. They found a group of 12 genes, all of which coded for cell-wall synthesis enzymes.

But there are still a lot of unanswered questions. What is the cseC actually sensing? What is the exact purpose of the cseA? Why produce both a sigma-factor and a heafty response pathway? Which intermediates are used for activating? And, most importantly, can we hijack this somehow to kill bacteria?
I can't wait to get to work with it :D
---
Hutchings, M., Hong, H., Leibovitz, E., Sutcliffe, I., & Buttner, M. (2006). The E Cell Envelope Stress Response of Streptomyces coelicolor Is Influenced by a Novel Lipoprotein, CseA Journal of Bacteriology, 188 (20), 7222-7229 DOI: 10.1128/JB.00818-06
Hong, H., Paget, M., & Buttner, M. (2002). A signal transduction system in Streptomyces coelicolor that activates the expression of a putative cell wall glycan operon in response to vancomycin and other cell wall-specific antibiotics Molecular Microbiology, 44 (5), 1199-1211 DOI: 10.1046/j.1365-2958.2002.02960.x
Jacobs C, Frère JM, & Normark S (1997). Cytosolic intermediates for cell wall biosynthesis and degradation control inducible beta-lactam resistance in gram-negative bacteria. Cell, 88 (6), 823-32 PMID: 9118225




















